23+ Atoms To Moles Calculator
A mole according to the International System of Units is a chemical substance with exactly 602214076x1023 Avogadros. Check how moles are relevant for solutions with our molarity calculator.
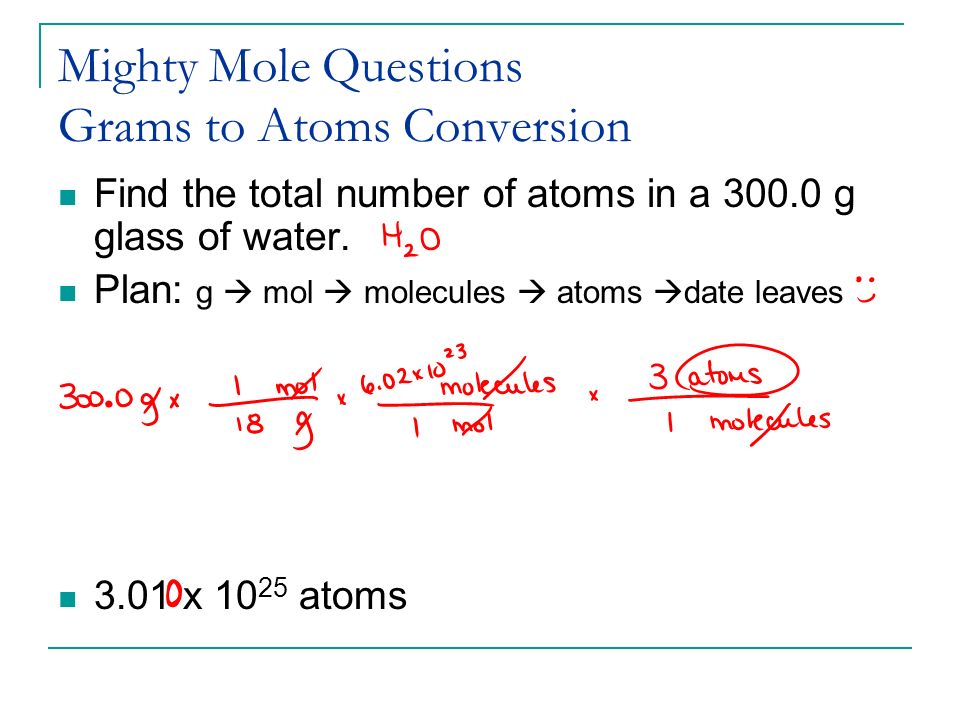
Unit V The Mole Concept Ppt Video Online Download
How to add Chemistry Moles Calculator to my website.
. The mole abbreviated mol is the SI unit of amount of substance. We assume you are converting between atom and mole. How do you go from grams to molecules.
Then the number of moles of the substance must be converted to atoms. While the mass of 1 mole of a particular substance will vary 1 mole of ANY substance will ALWAYS have approximately 602x1023 atoms rounded to 3 significant figures. Mass of the material Molecular Weight of the material Press the Calculate button.
To convert atoms to moles you need to use a calculator. Calculate he number of moles you have by taking the. To convert moles to atoms you can use a calculator or a simple formula.
We assume you are converting between atom and mole. Molecules To Moles Calculation. It can be used as a conversion factor from atoms to moles or moles to atoms.
First enter the number of atoms you have. To calculate the moles from grams of a substance we need the molar mass of the substance. One mole of helium gas is equal to.
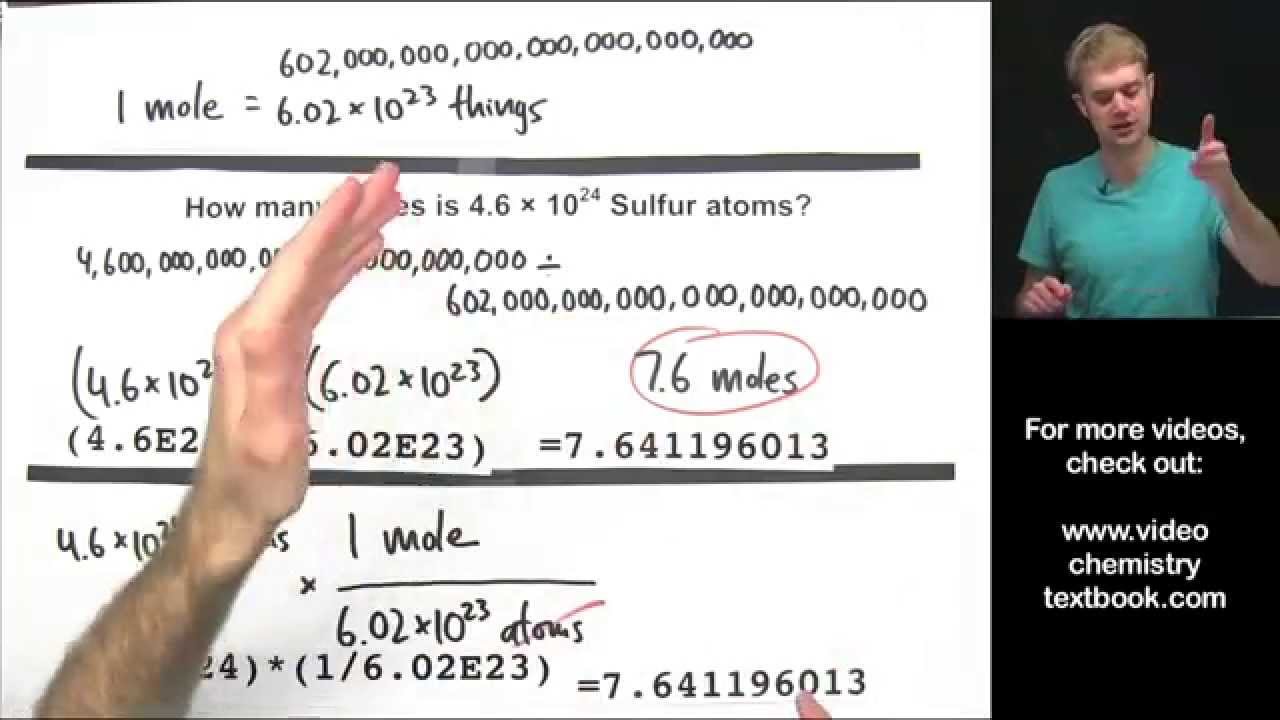
There are 6022x10 23 atoms in 1 mole of atoms. This number is also referred to as Avogadros number. This number is called.
The answer is 60221415E23. How do you calculate grams from atoms. Mole Molecules 60221415 x.
This calculator calculates the mole using molecule values. Then press the button that says Convert The calculator will then show. You can view more details on each measurement unit.
How do you convert grams to atoms calculator. How many atom in 1 mole. One mole is defined to contain exactly 602214076 x 10 23 elementary entities atoms molecules ions or electrons.
Avogadros Number 6022x10 23. Select Moles from the drop down menu titled Calculate. To use the calculator simply enter the number of moles and click calculate The answer will be in.
A mole of an element or molecule contains exactly 60221407610 23 particles. You can view more details on each measurement unit. The answer is 60221415E23.
A mole of a substance is defined as the amount of that substance that contains as many elementary entities as there are atoms in 0012 kilogram of carbon-12. Converting moles of a substance to atoms requires a conversion factor of Avogadros. How many atoms in 1 mole.
Mass Moles Conversions Ag Environmental Sciences Academy

Avogadro S Number The Mole Grams Atoms Molar Mass Calculations Introduction Youtube
Moles And Percents Wyzant Lessons

Moles To Atoms Calculator Calculator Academy
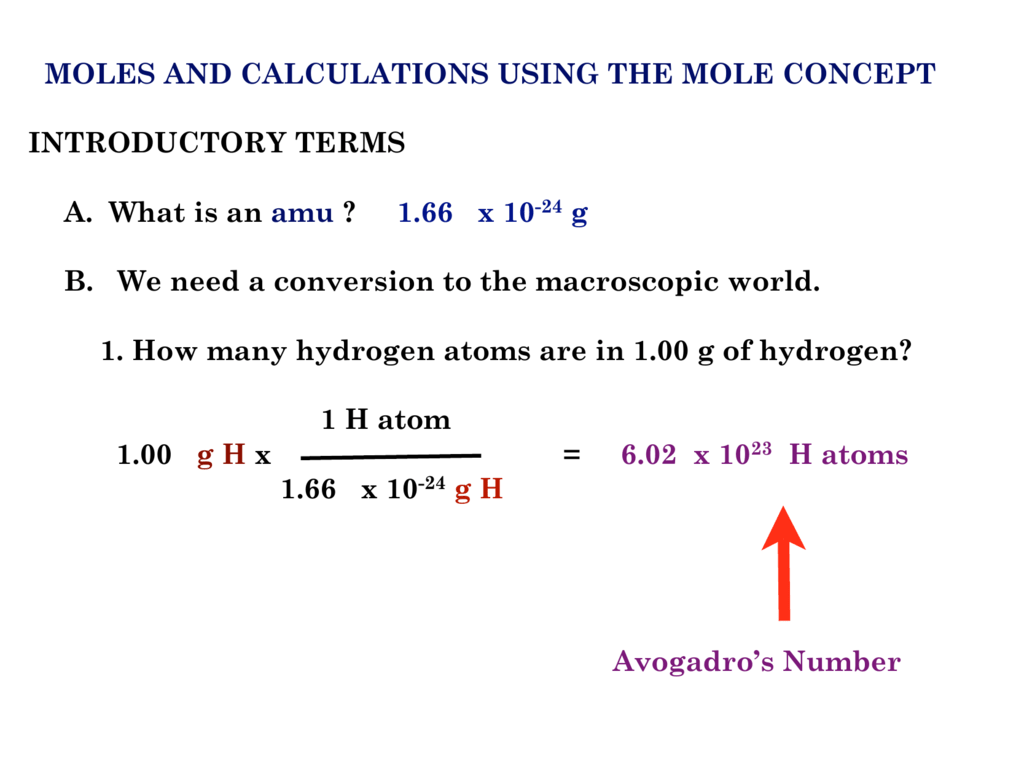
Moles And Calculations Using The Mole Concept

How To Convert Atoms To Grams Sciencing

Full Pdf Pdf Chemical Elements Atoms

Unit V The Mole Concept Ppt Video Online Download

Moles To Atoms Calculator Calculator Academy
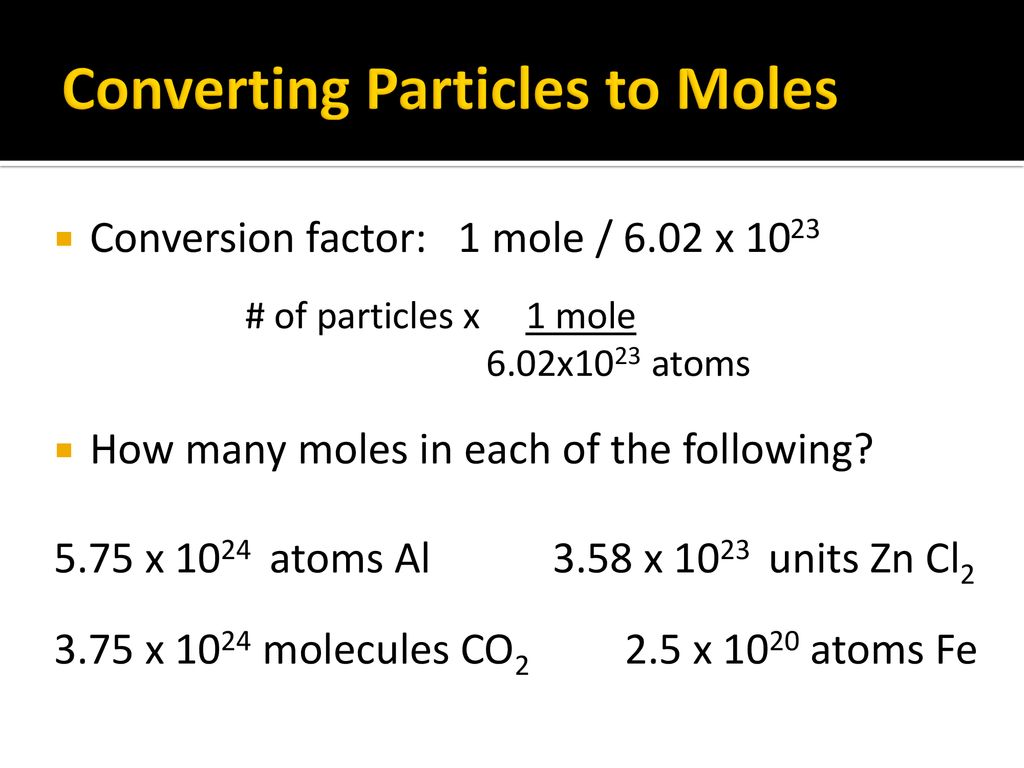
Chapter 11 The Mole Ppt Download
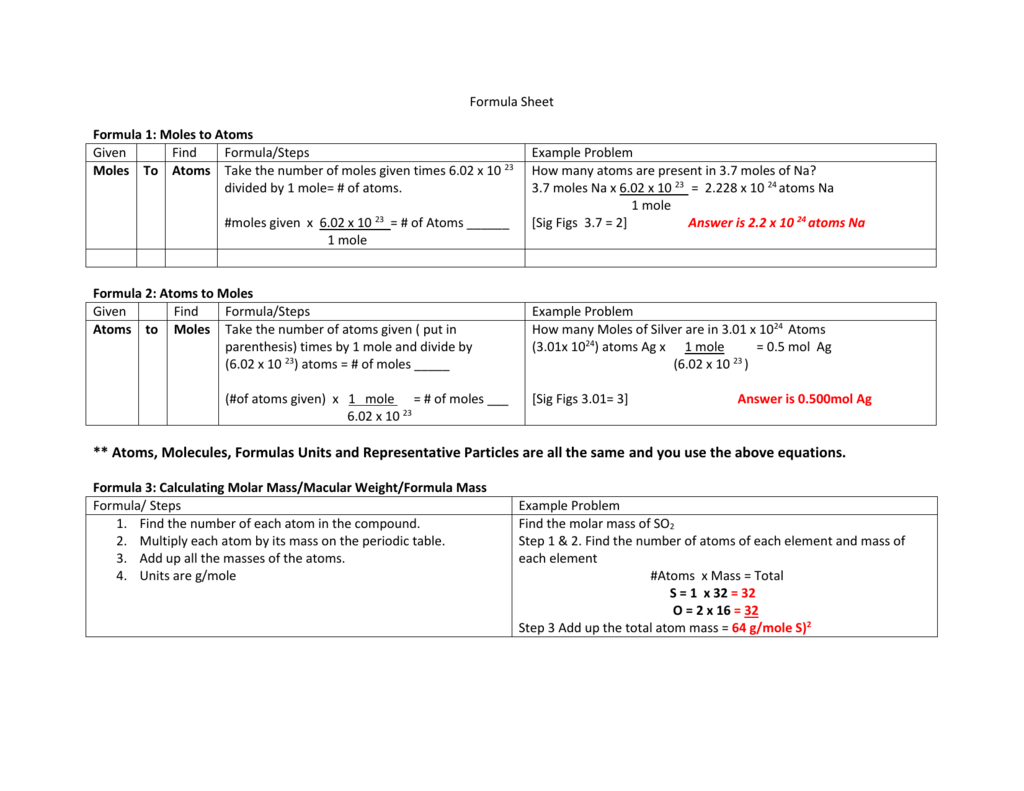
Formula 1 Moles To Atoms

How To Convert Moles To Number Of Molecules Youtube

Mole Calculator Conversion Between Molar Mass Moles

Atoms To Moles Calculator Dbcalculator Com

Gaseous State
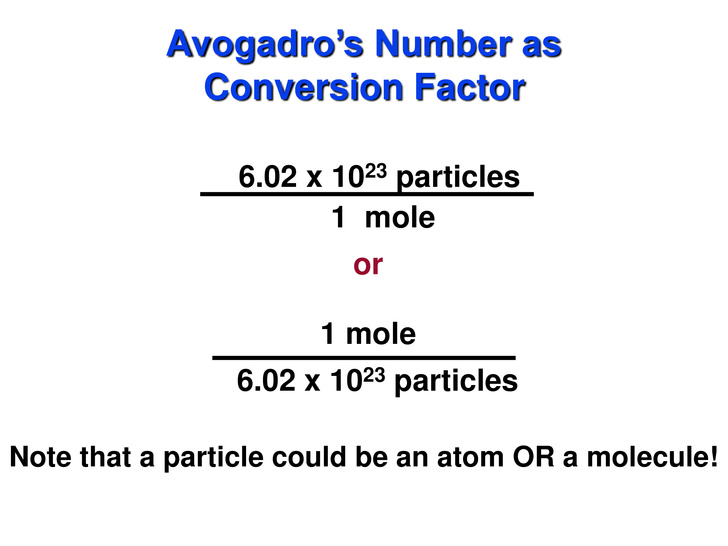
How Many Molecules Are In 2 00 Moles Of No 2 Socratic

Atoms To Moles Calculator Convert Moles To Atoms Atoms To Moles